
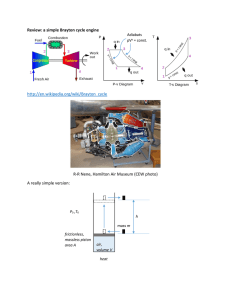
take some work out of the air and use it to drive the compressor, and.Turbine and exhaust nozzle, with which we c - d Adiabatic, quasi-static (or reversible) expansion in the.b - c Constant pressure fuel combustion (idealized as constant.a - b Adiabatic, quasi-static (or reversible).The cycle consists of four processes, as shown inįigure 3.13 alongside a sketch of an engine: The Brayton cycle (or Joule cycle) represents the operation of a gas 3 Brayton Cycle for Jet Propulsion: the Ideal Ramjet 2 Gas Turbine Technology and Thermodynamics The First Law Previous: 3.6 Diesel Cycle Contents Index This will be close to -445-40 next if we have to calculate for efficiency, If we have to calculate for efficiency this is work upon which work is 80 John Which is 620 Joel this is 0.129 It will solve efficiency of car note between T24 it will find efficiency of car note 2024 This is 1 -2C by th this is one -37 Kelvin By 851 Kelvin this will be 0.565.Next: 3.8 Muddiest points on Up: 3. It will write kOC this is equals to NCP The 4 -3. 31 from here we will get 851 Calvin minus 4 to 5 Calvin.
#NET WORKDONE IN BRAYTON CYCLE PLUS#
It will solve this is 0070 In 23 x two Plus 1. Here we will get physicals too and CPI in two T 2 -1 1. And if we solve for D we will say total work is 2 50 down Plus 96 John -216, John -40 Year John this will be equals 2 82 john this will be equals 2 82 john Now it will solve this four E. This will be close to -3 x two -0.070 into 8.31 changing temperatures 4-5 Kelvin -370 Calvin Physical, Sue minus 48. We will get minus three by 20 point 070 into 8.31 into 741 Kelvin -851 Kelvin This will be close to 96 jobs.
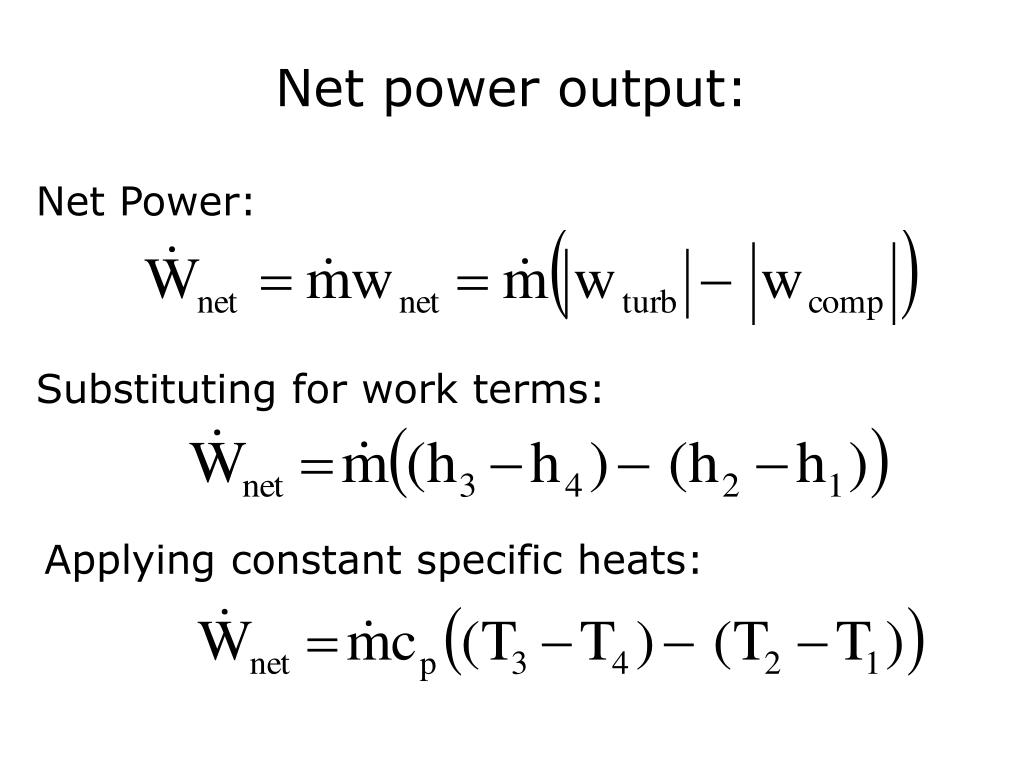
Here, it will find work done from 2 to 3 here we will get work done from 2 to 3. It will fall for c part, it will fall for C. The food, this is the food be fought by ending to our This will be equals to 365 Kelvin. It will put all the values We will get 851 Calvin It will be three. We will solve for old cycle for sleep ability one, this is P one V one by and we will put all the values we will get, this is 4 to 5 kelvin. We know that he is the girls to PV by anna. Even after we will put all the values we will get this minus 216 jumps now in the second option we will fall for T. It will solve this from 1 to 4, this is P three, this is B four minus. If we will solve this here, we will get one liter -0.50 later After here, this is tender Super -3 disease 2 50 later, this is 2 50 later, it will solve this fourth state 1 to 4.
This is 1.01 into 10 days to power five pascal per 80 m. It will be even we too minus we even here we will be 4.918 AM. Because P and Delta B are known for both displacements. Both B and delta B are known for both displacement for both displacement. The work can be calculated because both because both B and better to be known. Now we will fall this, we will we know that firstly we will fall for a part here. We have to determine these some of the terms. We have given the data foot pressure and volume we have to determine involved in temperature. Hello everyone let us do the following question. Calculate and compare to the efficiency of a Carnot engine running between the same high and low temperatures. In a real Brayton engine will reduce this ideal efficiency. Determine the efficiency for this Brayton engine (Eq.

The Brayton cycle shown in Figure $\mathrm+R$į.


 0 kommentar(er)
0 kommentar(er)
